Last Revised: January 20, 2026
The use of biological materials in laboratories must be registered with EHRS by completion of the Biological Registration in BioRAFT. The Principal Investigator or the Lab Safety Coordinator may complete the General Biological Usage Survey (Biological Registration), but only the PI can certify it. For more information, see below.
To see a pdf of this process with screen shots, click on this link or see the information below.
The purpose of the Biological Registration is to establish a comprehensive database pertaining to biological research at Penn. The information collected will be used to conduct risk assessments for work with biological agents and to assist EHRS and Penn faculty with their compliance efforts.
- The Biological Registration in your lab profile must be updated annually or as changes occur.
- Principal Investigators are responsible for ensuring that the submission is complete and accurately represents current research efforts.
- The task of completing the Biological Registration can be delegated to an informed and capable lab member who is given permission in BioRAFT to complete the registration.
- Principal Investigators must review and certify the Biological Registration prior to submission to EHRS for review.
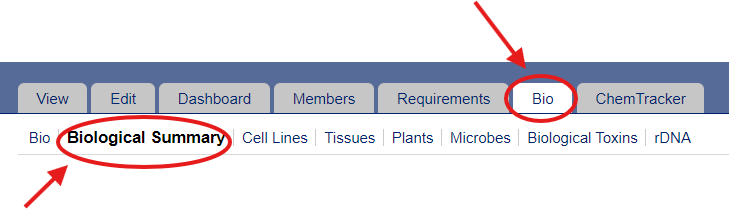
1) To complete your lab’s biological registration, navigate to the “Bio” tab in your BioRAFT profile. Use either the pop-up Wizard or click on “Biological Summary” to access the General Biological Usage Survey. Click “Edit Responses” in the General Biological Usage Survey to edit the requested information.
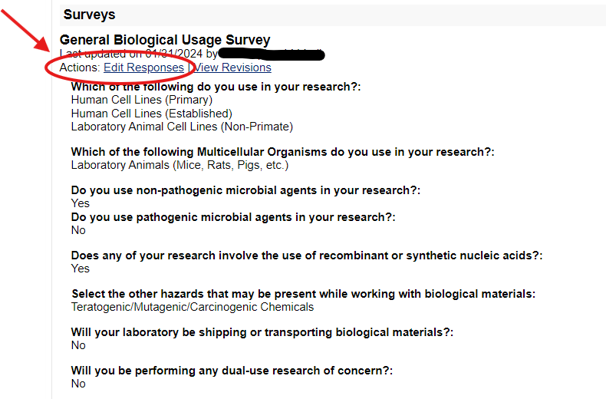
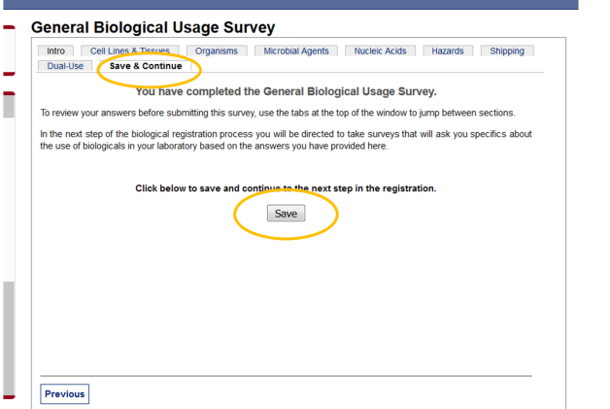
2) Go through the survey tabs to confirm the information is correct. When you are finished looking through the information in the various surveys, click on the “Save and Continue” tab, and then click “Save.”
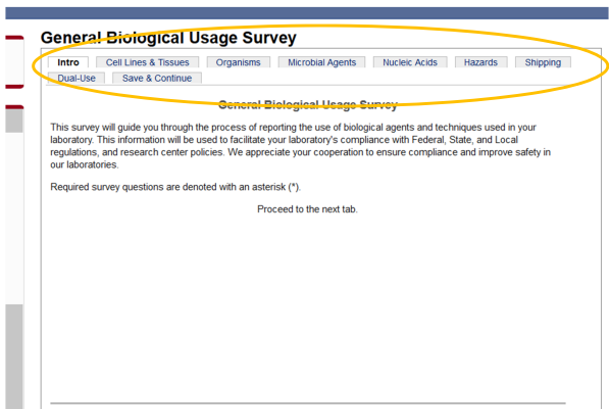
3) Review any additional surveys that may have been completed, such as the Microbial Agents Survey and Biological Toxins Survey. Then, review the Source Materials table to confirm all biological agents that are in the lab are represented in the table.
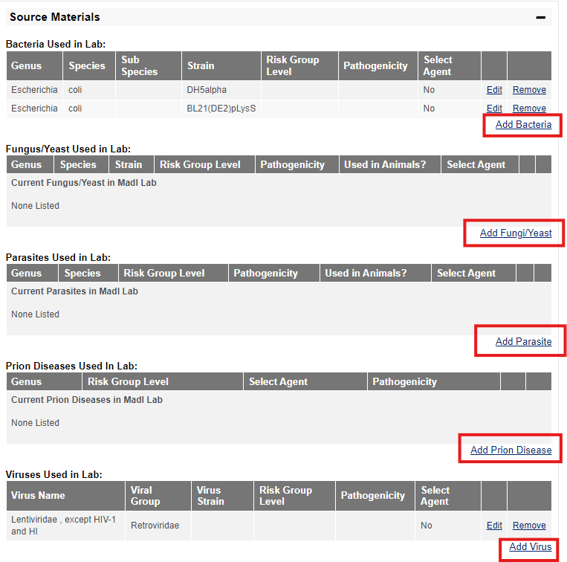
4) Once you have completed all the required information, “SUBMIT” the survey. The PI of your lab will receive an email notification to this fact. The PI must then go into BioRAFT to the “Bio” tab and certify the accuracy of the information that was entered. The certification of the Biological Registration can only be completed by the principal investigator of the lab.
If the Lab Safety Coordinator completes the Survey, the Principal Investigator will receive an email containing the following text:
---------------------------------------------------------------------------------------------------------------------
Please review and submit your Biological Registration to EHRS.
All information regarding your biological research has already been entered into the BioRAFT system.
It is now ready for your review and approval.
==========================================
What you need to do:
1. Follow the link below to your lab's online Biological summary
2. Review your lab's Biological Summary
3. Certify & Submit it to EHRS for review.
Follow this link to begin: https://penn.bioraft.com/node/######/biologicals/bioSumary
After you complete, an EHRS Biosafety Officer will review your Registration and contact you with any questions or concerns
---------------------------------------------------------------------------------------------------------------------
Follow the link in the email to the Biological Registration page, review the biological material selected and, if accurate, click submit located at the bottom of the page. If incorrect, edit the biological material selected before clicking submit. This will submit the Biological Registration to EHRS for review.