Last Revised: September 27, 2024
All research labs with personnel who have the potential to be exposed to bloodborne pathogens or human source material, including primary and established cell lines, blood products, body fluids, and tissue, must complete and review a lab specific Exposure Control Plan. Exposure Control Plans must be kept on file with EHRS and are attached to the lab's BioRAFT profile where it is available to all lab members.
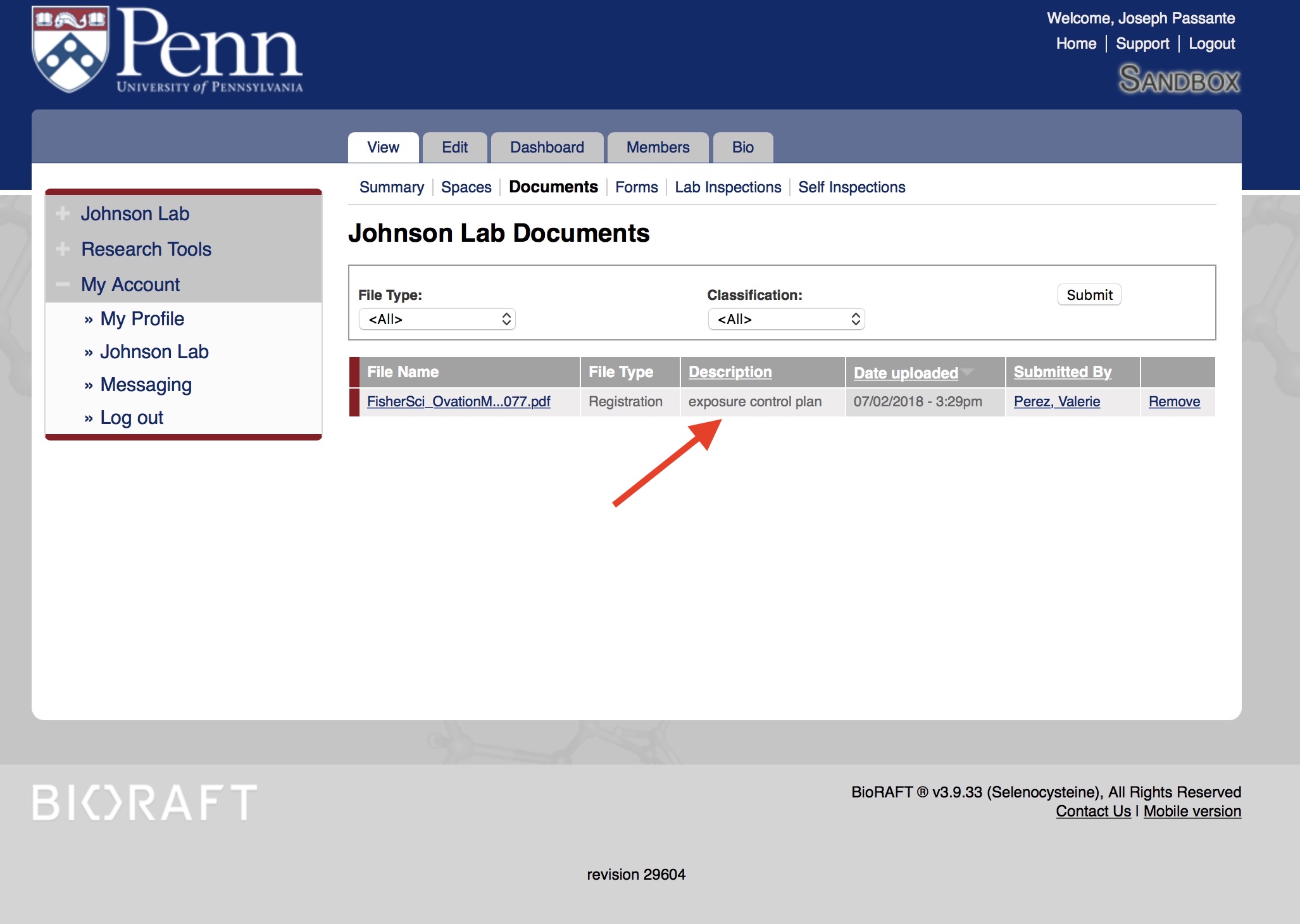
The Exposure Control Plan must be updated annually or whenever changes occur in the lab, such as new lab members or location. To check the date of your lab's exposure control plan, you must log into BioRAFT. Click on "View Lab Profile:"
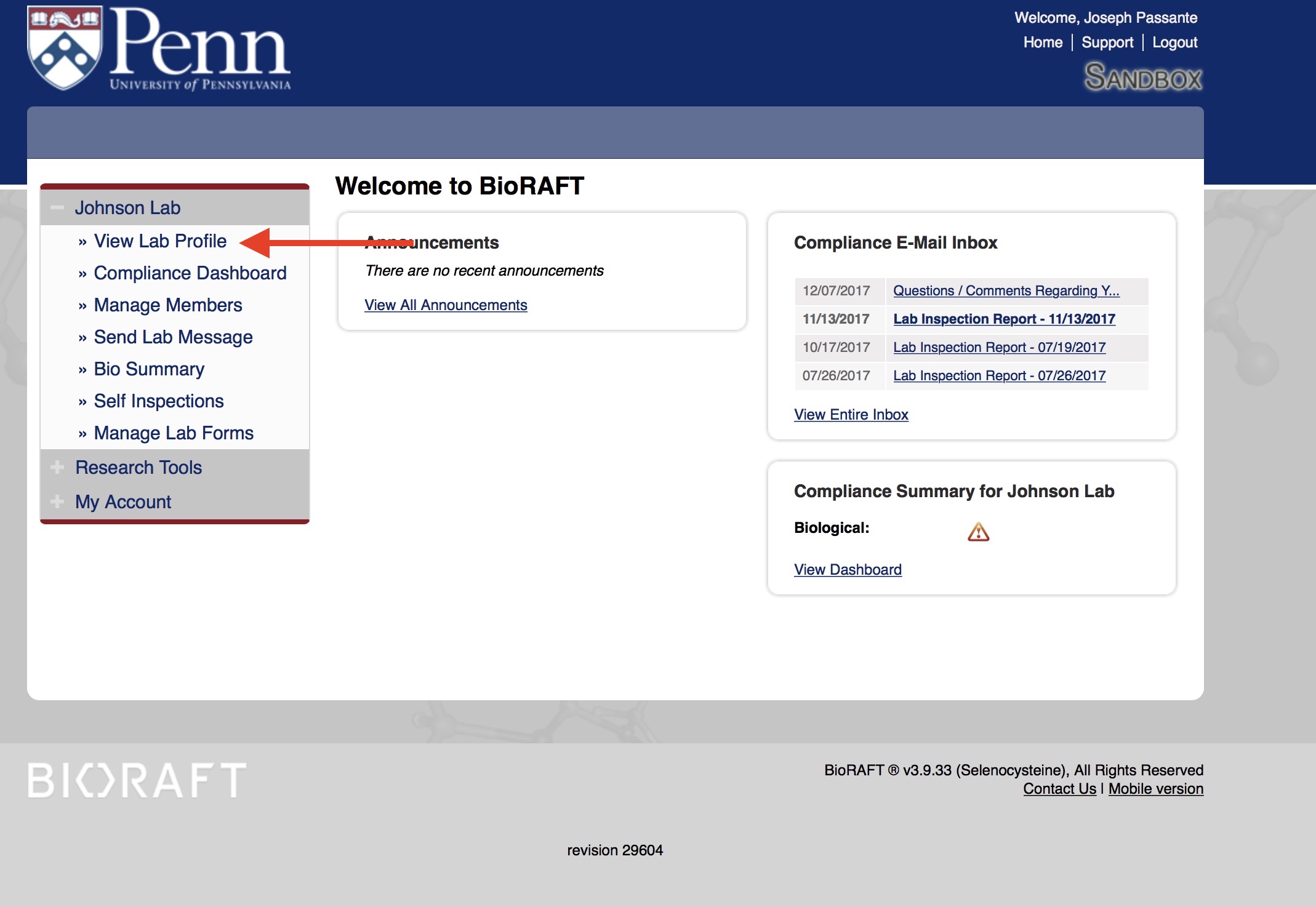
Then click on the "Documents" tab:
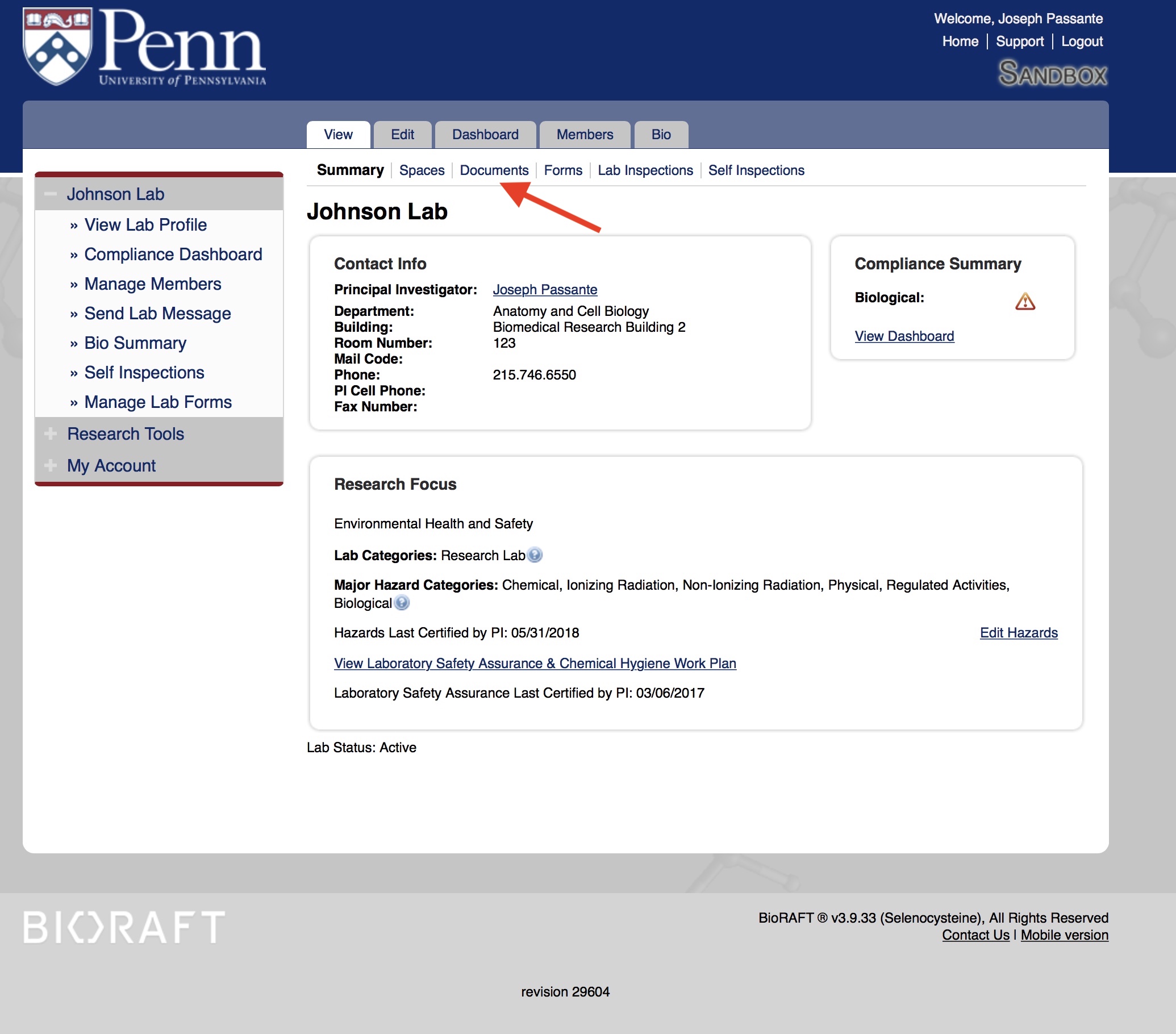
Look for a document in the window with the title "ECP" or Exposure Control Plan. If you do not see an Exposure Control Plan, you must complete and review a lab specific one. If the plan is more than a year old and there are no changes, you may submit last year's plan with a current date and an updated signature page. PIs and LSCs can upload ECPs directly to the lab's "Documents" section in BioRAFT. Upload the document as a "general" file type using the suggested naming convention "Lab Name_ECP_Date of Signatures." The document will be available to all personnel listed in the "Lab Members" section of the lab's profile.